Magnesium oxide (MgO) is a white, hygroscopic solid mineral that has gained significant attention in the pharmaceutical industry. Known for its diverse functionality, magnesium oxide is not only used as a dietary supplement but also plays a vital role as an excipient in drug formulations. Its wide range of applications stems from its chemical stability, alkaline nature, and bioavailability, making it a valuable ingredient in both prescription and over-the-counter (OTC) medications.
Pharmaceutical-Grade Magnesium Oxide
Pharmaceutical-grade magnesium oxide is manufactured to meet specific purity standards and is free from harmful contaminants. Its primary functions include acting as an antacid, a laxative, and a magnesium supplement. Additionally, it is used in tablet formulations for its compressibility and as a buffering agent.
Pharmaceutical-Grade Magnesium Oxide Production: A Scientific Overview
Magnesium oxide (MgO) is a versatile inorganic compound widely used in the pharmaceutical industry due to its antacid, laxative, and mineral supplementation properties. Producing high-purity, pharmaceutical-grade MgO requires a precisely controlled process to meet stringent regulatory standards, including those outlined by pharmacopeias such as USP, EP, and JP.
Below is a comprehensive description of the industrial process involved in the production of pharmaceutical-grade magnesium oxide:
Raw Material Selection
The production process begins with the selection of high-quality raw materials. Two main sources are used:
Natural minerals: Primarily magnesite (MgCO₃) or dolomite (CaMg(CO₃)₂).
Synthetic brines: High-purity magnesium salts such as magnesium chloride (MgCl₂) derived from seawater or underground brine deposits.
The selected raw materials must be free of heavy metals and other contaminants, as these can compromise the purity required for pharmaceutical applications.
Purification and Pre-treatment
When using synthetic brine, the brine undergoes several purification steps:
Precipitation of impurities: Addition of reagents like sodium hydroxide or calcium hydroxide to remove calcium, iron, or sulfates.
Filtration: To eliminate solid residues and ensure clarity.
Concentration: The purified brine may be concentrated through evaporation to increase magnesium content.
If magnesite is used, it is crushed, washed, and screened to obtain uniform particle size and remove unwanted materials.
Calcination (Thermal Decomposition)
The heart of MgO production lies in the calcination process, where magnesium carbonate (MgCO₃) is thermally decomposed to yield magnesium oxide (MgO) and carbon dioxide (CO₂):
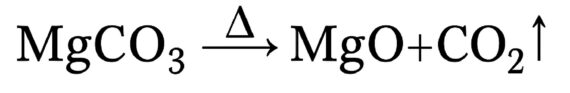
This reaction typically occurs at temperatures between 800°C and 1,000°C, depending on the desired reactivity and surface area of the final product.
Low-Temperature Calcination (Light MgO): Produces a more reactive, fine-powdered magnesium oxide with high surface area. This form is ideal for pharmaceutical applications.
High-Temperature Calcination (Dead-Burned MgO): Results in a less reactive product, more suited for industrial or refractory uses.
Cooling and Milling
After calcination, the hot MgO is rapidly cooled to prevent agglomeration and preserve its reactivity. The cooled material is then Milled into fine powder.
Classified using air separators to obtain uniform particle size suitable for oral or tablet formulations.
Purification (Post-Calcination)
To ensure pharmaceutical purity, the MgO may undergo further treatments such as:
Acid washing: To remove residual impurities.
Re-precipitation and filtration: In some processes, MgO is converted to magnesium hydroxide (Mg(OH)₂) and then re-calcined for enhanced purity.
Drying: Ensures minimal residual moisture content.
Quality Control and Compliance Testing
Every batch of pharmaceutical-grade MgO is subjected to rigorous analytical testing, including:
Purity assay (typically >98.0%)
Heavy metals screening (As, Pb, Cd, Hg)
Loss on ignition
Acid neutralization capacity
Particle size distribution
Microbial limits (especially for oral use)
Testing is performed in accordance with pharmacopeial standards (e.g., USP/NF, EP, JP), and each lot is issued with a Certificate of Analysis (CoA).
Packaging and Storage
The final product is carefully packaged in moisture-resistant, inert containers, often lined with polyethylene to prevent atmospheric absorption, as MgO is hygroscopic and can react with moisture and carbon dioxide in the air.
Storage conditions are typically:
Dry
Cool (below 25°C)
Protected from CO₂ exposure
As a Magnesium Supplement
Magnesium is an essential mineral involved in over 300 enzymatic reactions in the human body. It is crucial for muscle function, nerve transmission, blood glucose control, and protein synthesis. Magnesium oxide serves as a common oral supplement due to its high magnesium content by weight—approximately 60%.
Although its bioavailability is lower than other magnesium salts like magnesium citrate, its high elemental magnesium content makes it a cost-effective and efficient choice for correcting magnesium deficiencies, especially in long-term supplementation plans.
Use as an Antacid
One of the most traditional uses of magnesium oxide in the pharmaceutical sector is as an antacid. It neutralizes excess stomach acid, providing relief from conditions such as heartburn, indigestion, and acid reflux. In combination with other compounds such as aluminum hydroxide, it helps balance gastric pH and provides symptomatic relief.
Use as a Laxative
Magnesium oxide is often employed as a mild osmotic laxative. When ingested, it reacts with water in the intestines to form magnesium hydroxide, which draws water into the colon and promotes bowel movements. This makes it useful in treating occasional constipation. However, for chronic cases, other magnesium formulations might be preferred due to better absorption profiles.
Excipient in Solid Dosage Forms
Beyond its active pharmaceutical ingredient (API) roles, magnesium oxide also functions as an excipient in solid dosage formulations. It is used as:
A drying agent: due to its hygroscopic nature.
A bulking agent: to achieve desired tablet size and weight.
A pH adjuster: to maintain stability of pH-sensitive drugs.
Its role in maintaining the structural integrity of tablets and capsules is especially valuable in high-speed manufacturing processes.
Stability and Compatibility
Magnesium oxide is highly stable under a wide range of environmental conditions, which is critical in pharmaceutical applications. It does not easily degrade and can help stabilize sensitive active ingredients by buffering the microenvironment within tablets or capsules.
However, due to its basic pH, compatibility testing is essential before combining it with acidic drugs, as unwanted chemical reactions may occur that reduce efficacy or create impurities.
Comparison with Other Magnesium Salts
While magnesium oxide has its advantages, especially in cost and elemental content, it is often compared with other magnesium salts such as:
Magnesium citrate: more bioavailable but more expensive.
Magnesium chloride: highly soluble and suitable for intravenous use.
Magnesium sulfate: used in emergency settings like pre-eclampsia.
Despite its lower absorption rate, magnesium oxide remains a popular choice in oral formulations due to its availability, safety, and multifunctionality.
Regulatory and Safety Aspects
Magnesium oxide is generally recognized as safe (GRAS) by the U.S. FDA when used within specified limits. It is approved in many pharmacopeias including the USP, EP, and JP. However, excessive intake may lead to side effects such as diarrhea, nausea, or abdominal cramping.
In patients with renal impairment, magnesium accumulation can occur, so caution is advised. Proper labeling and dosage instructions are essential to avoid adverse effects.
Industrial Demand and Supply
Pharmaceutical-grade magnesium oxide is typically produced from magnesite ore or seawater brines. The demand is steadily increasing due to the rising prevalence of gastrointestinal disorders and magnesium deficiency across the globe. With the growth of the nutraceutical and supplement industries, more companies are sourcing high-purity magnesium oxide for inclusion in health products.
Magnesium Oxide Supplier
In the competitive and sensitive pharmaceutical market, the quality and reliability of raw materials are critical. At Utah Trading LLC, we partner with trusted international manufacturers to supply high-purity pharmaceutical-grade magnesium oxide that complies with global standards such as USP and EP, along with full documentation.
Understanding the specific needs of the pharmaceutical industry, our goal is not only to ensure timely delivery but also to support our clients with technical guidance and comprehensive documentation to simplify and accelerate their approval and production processes.
Conclusion
Magnesium oxide stands as a cornerstone in the pharmaceutical industry due to its diverse functional roles, from serving as an essential mineral supplement to acting as a reliable excipient in complex drug formulations. Its stability, cost-effectiveness, and high elemental magnesium content make it an attractive option for both therapeutic and manufacturing applications.
Despite some limitations in bioavailability, ongoing innovations in formulation science continue to enhance its performance and usability. As demand for high-quality, compliant pharmaceutical ingredients grows, the importance of sourcing from trusted suppliers like Utah Trading LLC becomes increasingly evident. By providing pharmaceutical-grade magnesium oxide that meets rigorous global standards, we help ensure product efficacy, patient safety, and regulatory success—supporting the industry’s broader mission of delivering better health outcomes worldwide.



